Explore the Scientific R&D Software
Explore the Scientific R&D Software
 Explore the Scientific R&D Software
Explore the Scientific R&D Software 
Scientific intelligence platform for AI-powered data management and workflow automation

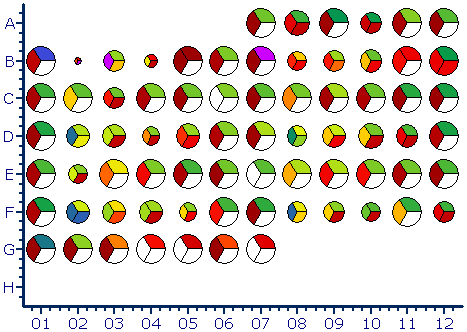
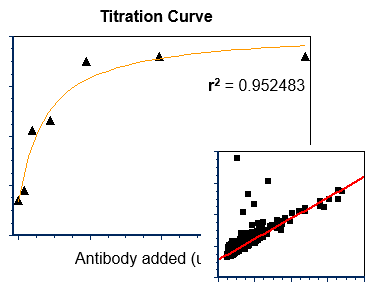
Statistical analysis and graphing software for scientists

Bioinformatics, cloning, and antibody discovery software

Plan, visualize, & document core molecular biology procedures

Proteomics software for analysis of mass spec data

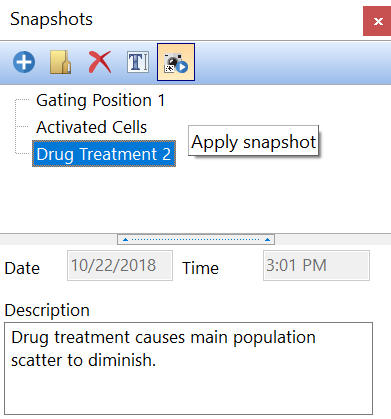
Electronic Lab Notebook to organize, search and share data

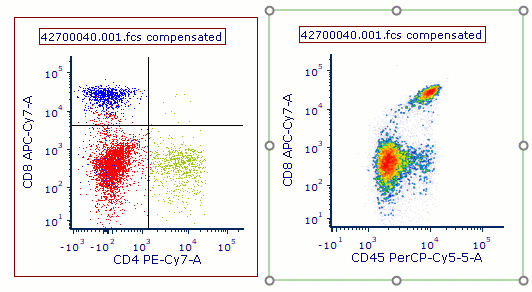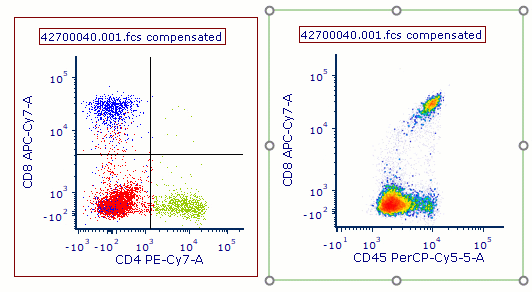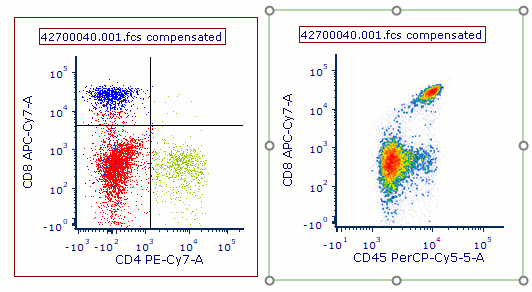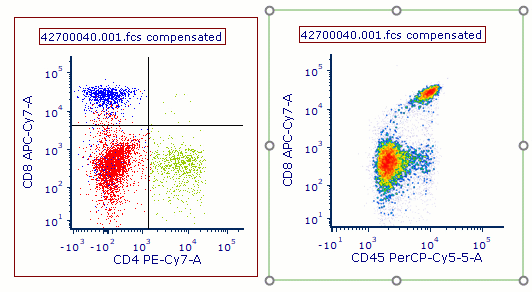
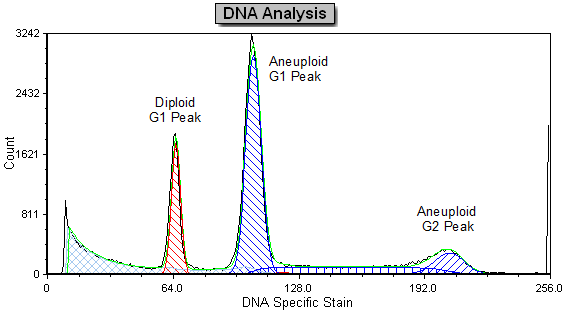
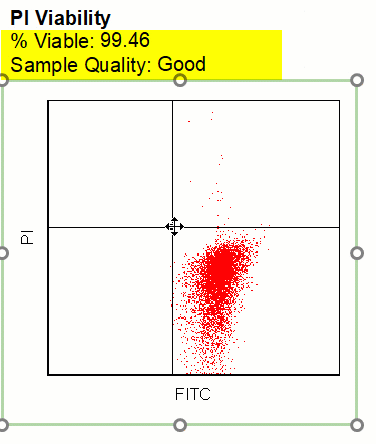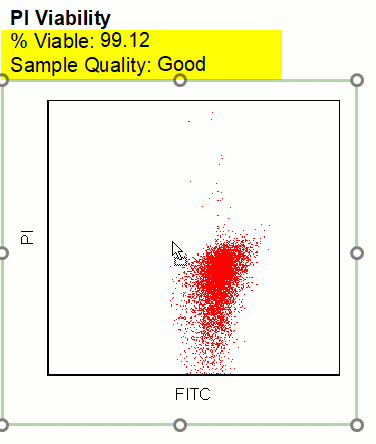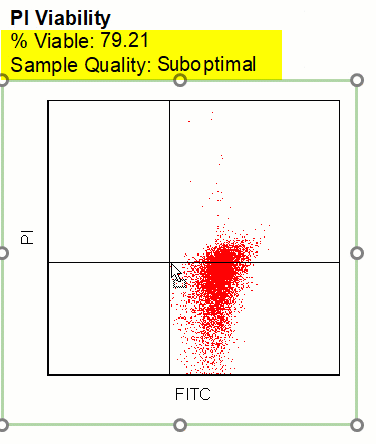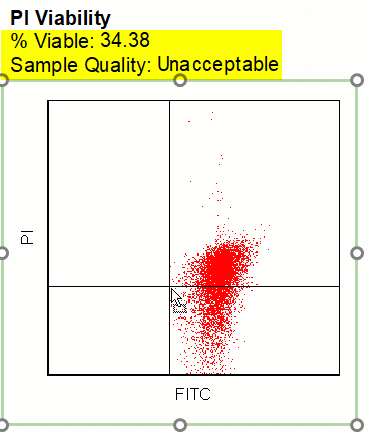
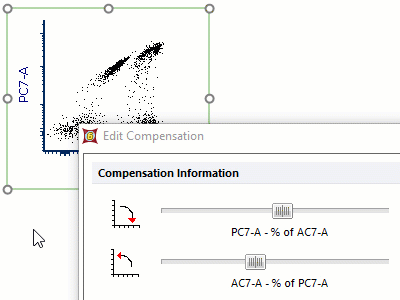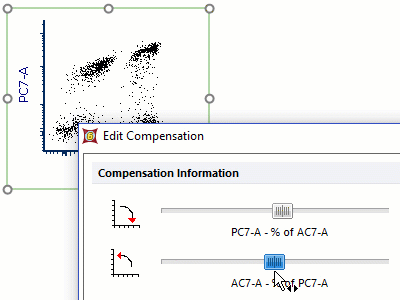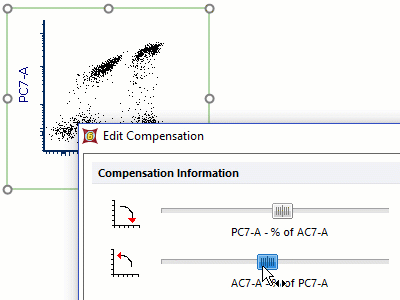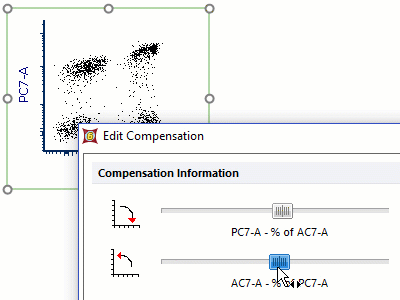
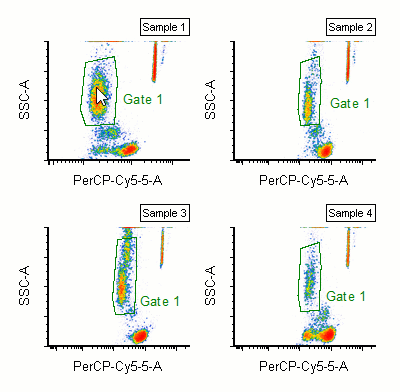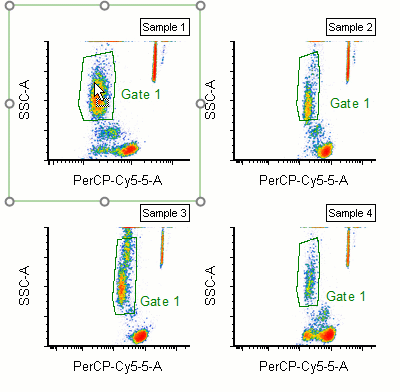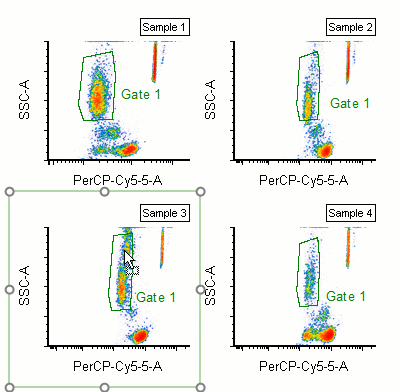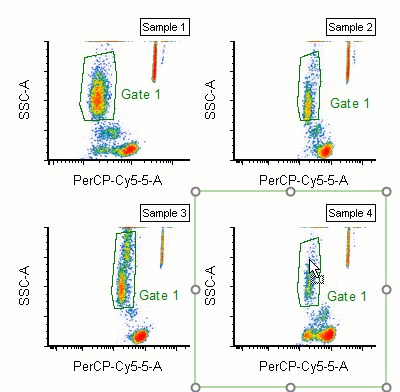
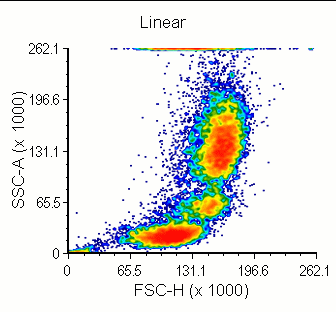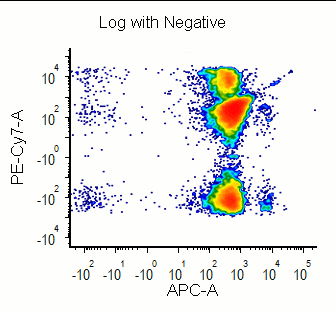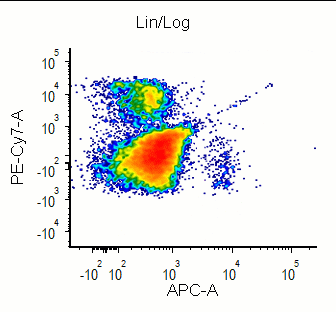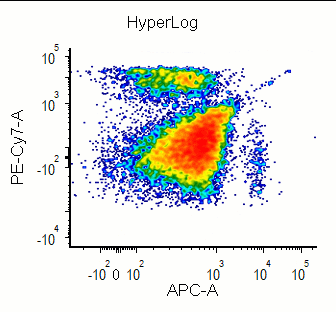
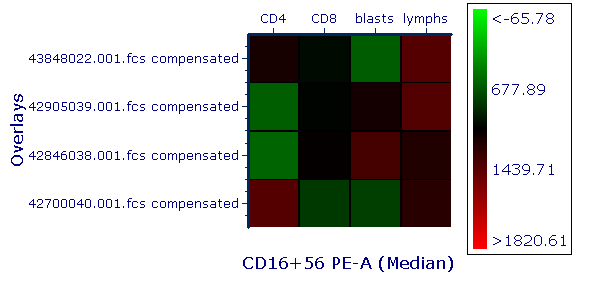
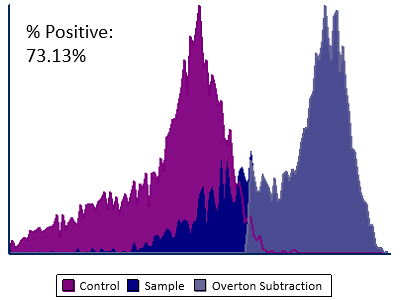
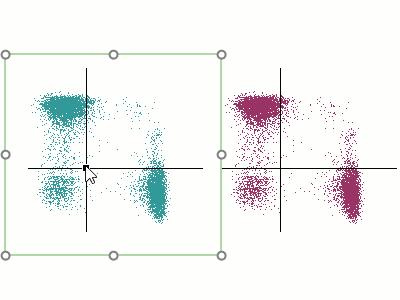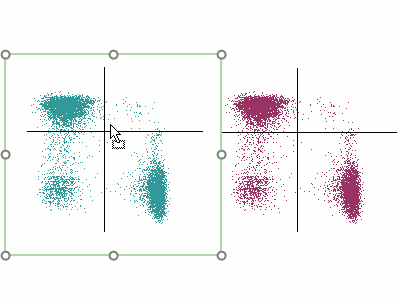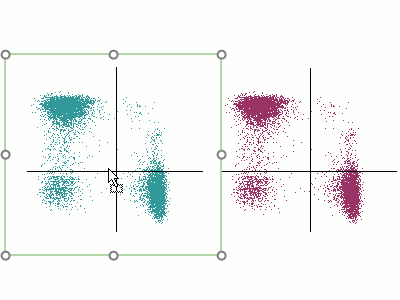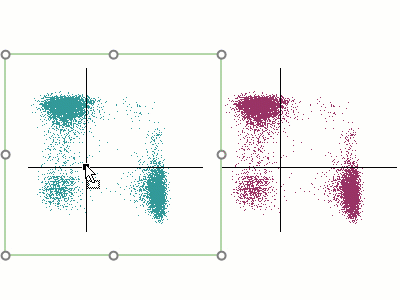
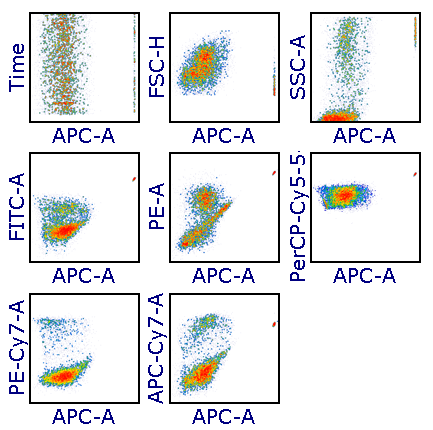
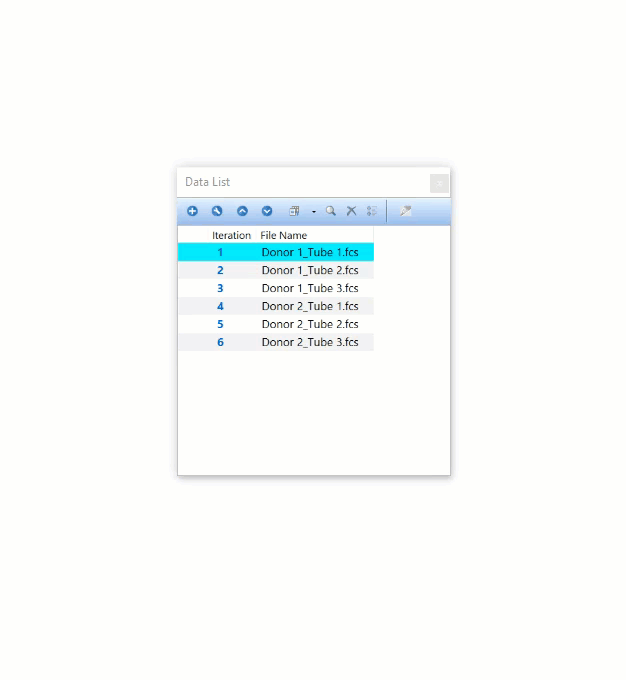
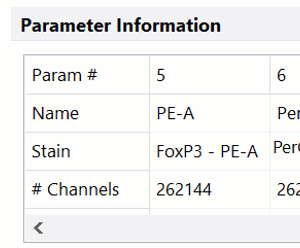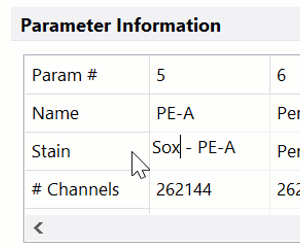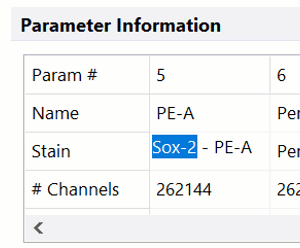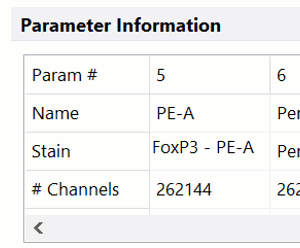
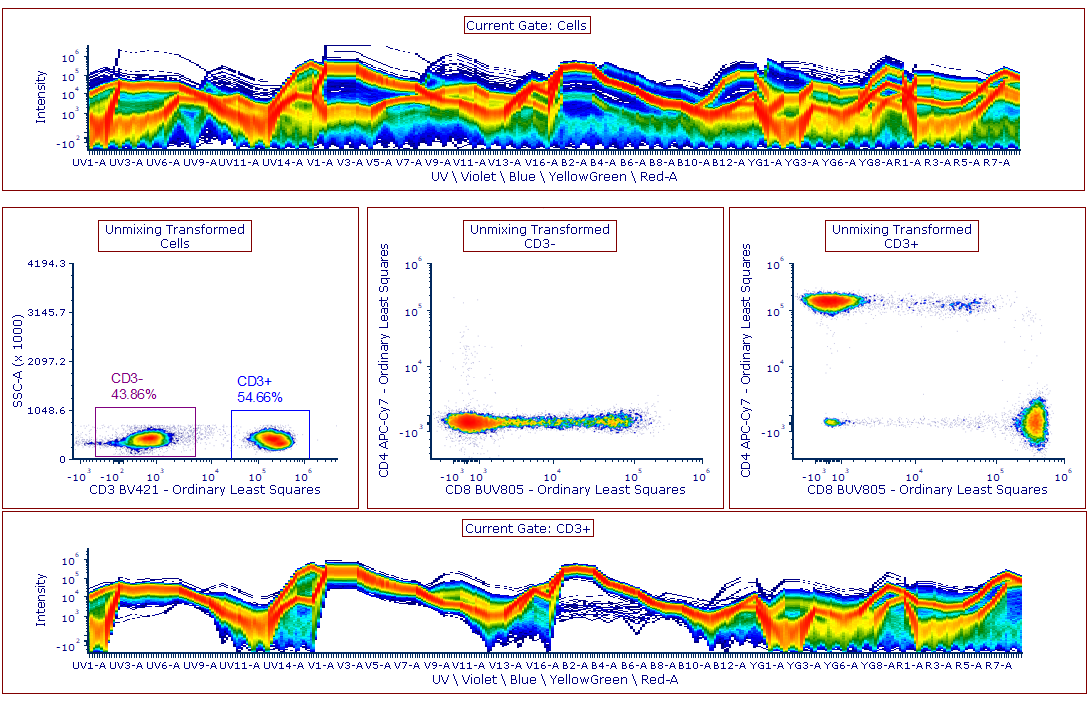
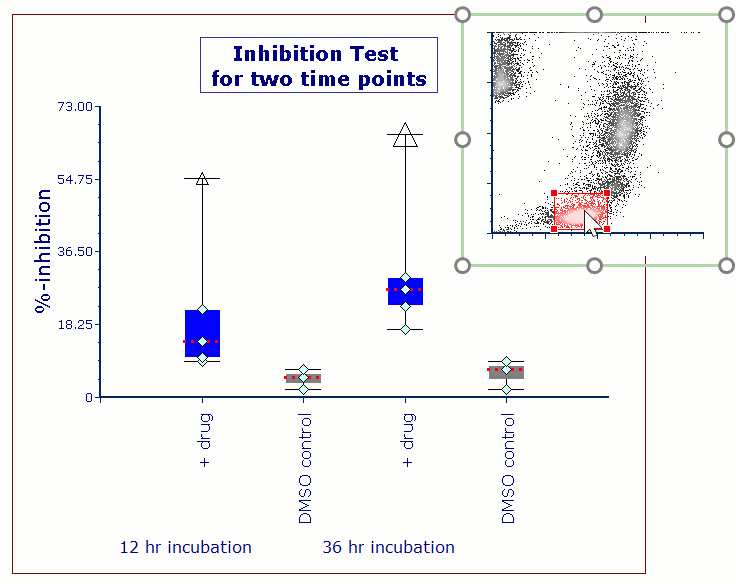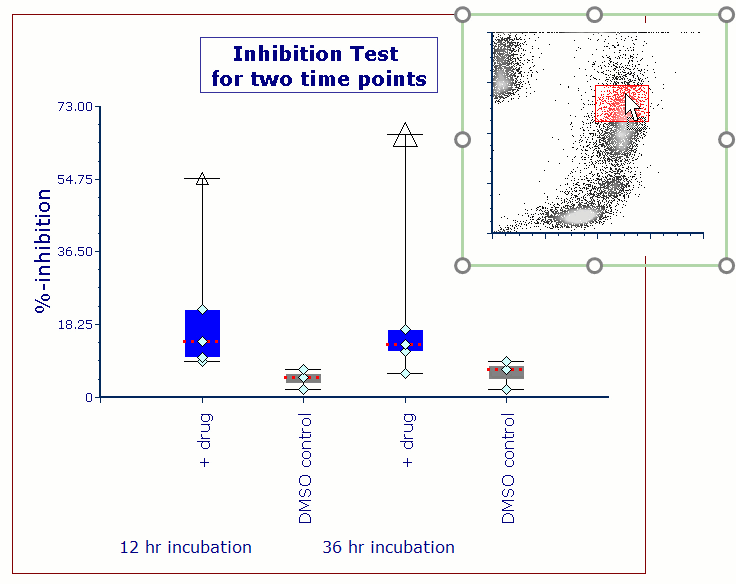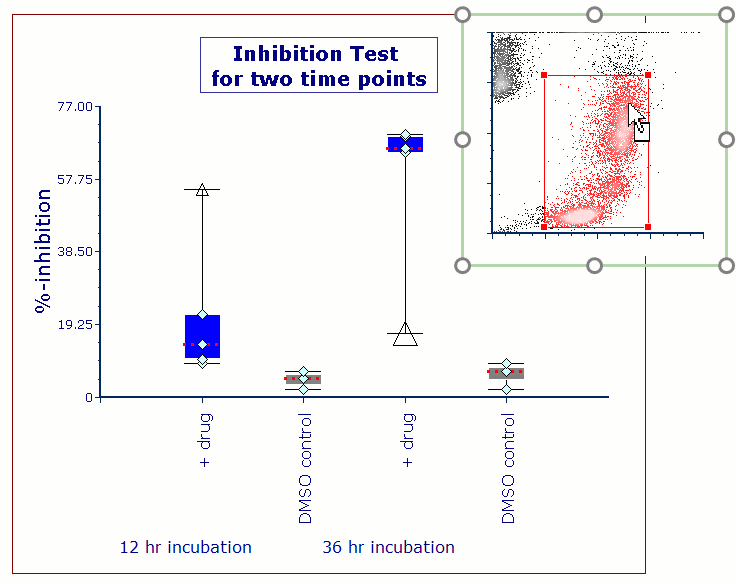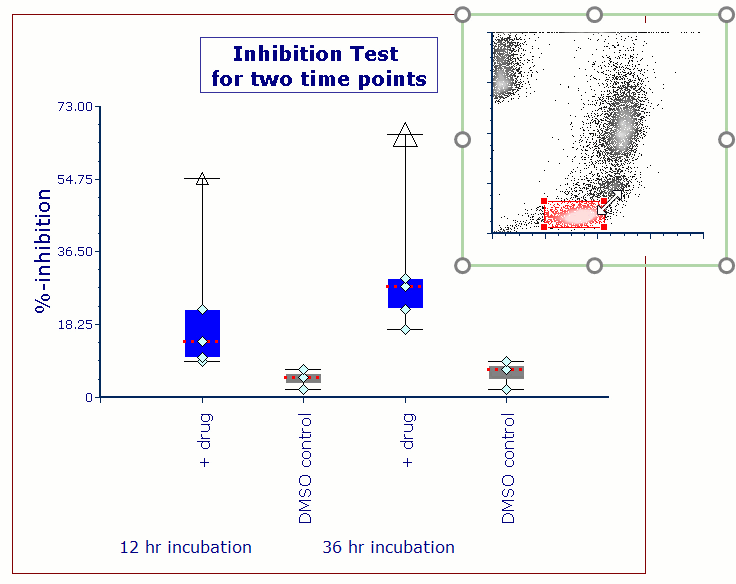
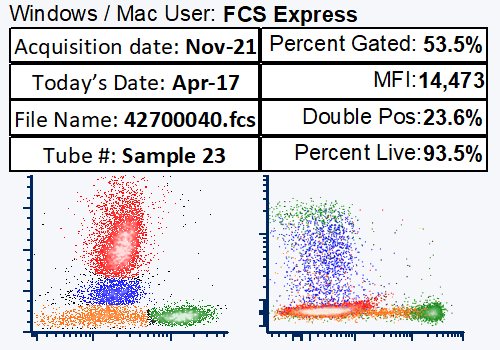
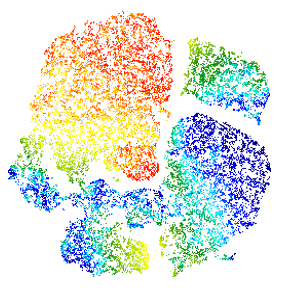
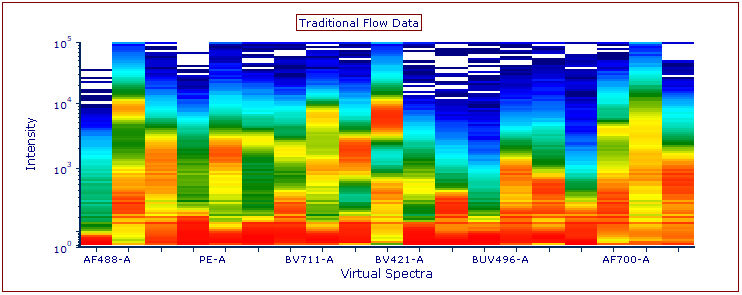
Modern cytometry analysis platform

Analysis, statistics, graphing and reporting of flow cytometry data

Intelligent panel design & inventory management for flow cytometry